The Inductive Effect Can Be Best Described as
Syed Salman Shafqat Lecture - 06 Fundamentals of Organic Chemistry 2 fInductive Effect f The inductive effect of a group of atoms is the change in the electron density at a nearby atom caused by differences in electronegativity. It is present in a σ bond unlike the electromeric effect which is present in a π bond.
The Wuzhi capsule showed a dose-dependent effect on tacrolimus pharmacokinetics demonstrating a stronger inhibitory effect than inductive effect.
. Inductive research involves the search for pattern from observation and the development of explanations theories for those patterns through series of hypotheses 2. An inductive load pulls a large amount of current when first energized then settles down to a full-load running current after a few seconds or cycles. D Inductive effects are always electron withdrawing.
When switched inductive loads can cause excessive voltages. Negative Inductive effect -I. The inductive effect I Effect reffers to the polarity produced in a molecule as a result of higher electronegativity of one atom compared to another.
Additionally this model can be used for individualizing. A deductive argument sets out to guarantee the truth of its conclusion based on the truth of its premises while an inductive argument attempts to offer a probability that its conclusion is true based on the truth of its premises. Many a times the same concept varies in names bw organic and inorganic chemistry.
The halogen atoms in alkyl halide are electron withdrawing while the alkyl. The effect of a group or atom of a compound in pulling electrons towards itself or in pushing them away. C A substituent that withdraws electron density from the ring will make the ring more reactive.
When the measurement is based on auto-balancing bridge method and the measured material is a bulky sample the capacitive coupling from sample to ground can cause some of these inductive effects. B If a substituent withdraws electron density from the ring it will be less reactive towards electrophiles. Inductive effects such as positive phase angles in impedance measurements have often confused the researchers when the material measured is nonmagnetic.
This deactivates the ring and is often referred to as a I effect. If the effect is permanent does that mean that even if the attached electron withdrawingdonating group is removed the induced effect still continues to exist. INDUCTIVE EFFECT 0Is defined as permanent displacement of shared electron pair in carbon chain towards more electronegative atom or group 2.
Some examples of inductive loads include transformers motors and wound control gear. Inductive approach also known in inductive reasoning starts with the observations and theories are proposed towards the end of the research process as a result of observations. A An atom or group can withdraw electron density from the ring by induction.
Inductive effects can be used to explain some aspects of organic reactions. A sigma bond between two atoms which differ in their electronegativity is polarized due to displacement of bond pair towards the more electronegative atom. Almost everywhere I checked the Inductive effect is described as a permanent effect with almost no mention of what that actually means.
Inductive Effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. INDUCTIVE EFFECT Inductive effect is defined as permanent displacement of shared election pair in carbon chain towards more electronegative atom or group. Inductive effect 1.
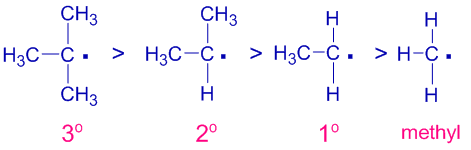
TYPES OF INDUCTIVE EFFECT 1. The more electronegative atom pulls the electrons. Hence you may consider inductive effect to be a kind of electronegativity effect.
This can also be called as permanent polarisation of single bonds. Inductive effect is an effect regarding the transmission of unequal sharing of the bonding electron through a chain of atoms in a molecule leading to a permanent dipole in a bond. Well inductive effective is a concept of organic chemistry.
The inductive effect can be electron-releasing or electron- withdrawing. This effect occurs due to the differences. Hyperconjugation is best described as.
For instance electron-withdrawing groups such as NO 2 CN CHO COOH and the halogens substituted on a benzene ring reduce the electron density on the ring and decrease its. Groups based on more electronegative atoms O F Cl may be σ-acceptors drawing electron density away from the ring via a simple inductive effect which arises from the electronegativity of the substituent. What exactly is permanent about the effect.
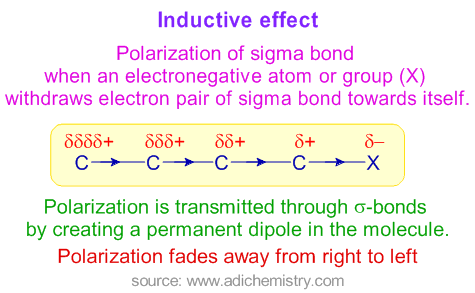
An inductive effect is an electronic effect due to the polarisation of bonds within a molecule or ion. In chemistry the inductive effect in a molecule is a local change in the electron density due to electron-withdrawing or electron-donating groups elsewhere in the molecule resulting in a permanent dipole in a bond. In addition to inductive loads there are resistance and capacitive loads.
This is typically due to an electronegativity difference between the atoms at either end of the bond. In this process the electronegative atom gets partial negative charge. This effect can arise in sigma bonds whereas the electromeric effect can only arise in pi bonds.
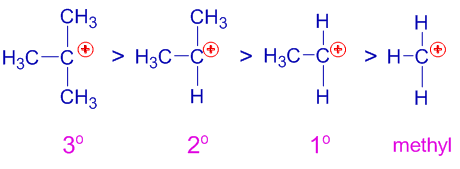
Alternatively it can be evaluated as a new parameter characterizing the substituent effect say a new scale of group electronegativity different from the suggested scales. Inductive effects are generally considered to be transmitted through the σ-framework of a molecule. A delocalization of pi electrons into a nearby empty orbital B delocalization of sigma electrons into a nearby empty orbital C the effect of alkyl groups donating a small amount of electron density inductively into a carbocation carbon.
This transmission of the charge finally leads to a fixed electrical charge on atoms. An inductive enumerative generalization moves from something we know about larger group the target to a claim about a smaller group the. The electronegativity of an element is usually a measure of its ability to attract σ-electrons2 In many synthetic operations selectivity reflects the energy differences between reagents activated intermediates and transition states.
The electron withdrawing nature of groups or atoms is called negative inductive effect. With two parameters the interaction of polar substituents is expressed with similar high precision R099 as with charged substituents and one parameter. Inductive effect is an effect caused by the transmission of an electrical charge throughout a chain of atoms.
The polarization of a σ bond due to electron withdrawing or electron donating effect of adjacent groups or atoms is referred to as inductive effect. Our model can accurately describe population pharmacokinetics of tacrolimus when combined with different doses of Wuzhi capsule. 1 f INDUCTIVE EFFECT CHEM1112 Dr.
Inductive Effect Positive Negative Definition Examples Applications

Inductive Effect Positive Negative Definition Examples Applications
Inductive Effect Positive Negative Definition Examples Applications

Comments
Post a Comment